The good news about nuclear bombs is that only two elements can be used induce a nuclear explosion: plutonium (Pu) and highly enriched uranium (HEU). While securing and detecting these materials is a herculean task, at least the field of materials is narrowed.
First, a refresher crash course in chemistry:
- An element is a substance that cannot be separated into any contributing substances. It is defined by its atomic number: the number of protons in the nucleus of one atom of the element (so Hydrogen=1, Oxygen=8, Uranium=92, Plutonium=94).
- The basic structure of an atom is the nucleus, and electrons zipping around the nucleus. The electrons take up most of the space of the atom–think a football field (the electrons) vs. a butterfly at the center of the 50 yard line (the nucleus). But the masses are flipped: the nucleus is almost 100% of all the mass in the atom.
- The nucleus is held together by the interplay between protons and neutrons. Because the electrons are so light, an atom’s atomic weight is the sum of its protons and neutrons. Elements can occur naturally with different numbers of neutrons; Uranium 238 is a Uranium atom with 146 neutrons; Uranium 235 has only 143 neutrons per atom.
- The higher the ratio of neutrons-to-protons in the nucleus, the less stable the atom. This is because the force of electrons is out-balanced by the weight in the nucleus. (Hydrogen is stable because the charge of one electron balances one proton; Uranium is unstable because the charge of 92 electrons must counter-balance 92 protons and 146 neutrons).
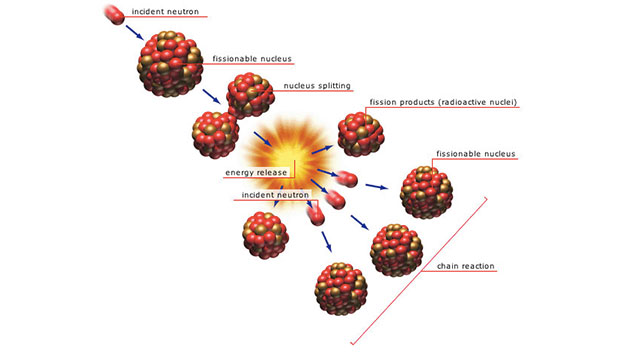
- When a stray neutron hits an unstable nucleus with sufficient force, the atom breaks apart. This process emits energy, as the forces that were holding the atom together now relax.
- Generally, when an atom breaks apart due to neutron bombardment, the components (electrons, neutrons, and protons) bond with other atoms to make different elements or compounds. However, when Uranium 235 and Plutonium are bombarded, two neutrons remain free, not taken up by other atoms. These neutrons then hit another nucleus, and the two neutrons resulting from that bombardment hit another nucleus.
- This “chain reaction”—neutrons breaking apart atoms that contribute two more neutrons that will then bombard additional atoms—can run away. The energy that is released from each atom comes together and the result is an enormous force that cannot be matched by regular explosives—a nuclear explosion.
Since Pu and HEU are the only materials that can generate a nuclear explosion, nuclear threat reduction efforts prioritize protecting and detecting these elements.
Silverside Detectors focuses on neutron detection. Since very few substances emit neutrons, this is the best way to reduce the number of false positives that detectors register. (Gamma ray detection is an important component of radiation detection systems, but since such innocuous materials as bananas and kitty litter emit gamma rays, the false-positives are distracting, and difficult and costly to minimize.)
Neutron detection can arrest illicit shipments of plutonium just by having detectors near the movement of the material, HEU, on the other hand, is more difficult, since in many situations it doesn’t emit many neutrons (and, as previously mentioned, it is difficult to detect its gamma ray signal as well). By leveraging larger surface area neutron detectors, larger coverage can detect the more faint HEU neutron signals, and a larger surface area detectors can be used in less radioactive so-called active interrogation systems (next up in this series: Nuclear Threat Reduction 101: Why surface area matters).
Neutron detection is just one component of a total threat reduction system, but a critical component for protecting our cities from a nuclear device.
Recent Comments